NEW
Cavex ProphyPaste
Cavex ProphyPaste is a pH neutral prophylactic paste containing fluoride, anti-staining and anti-bacterial components. Due to the ideal consistency it is easy to apply and does not splatter.
View product
Highlighted products

29 January ’24
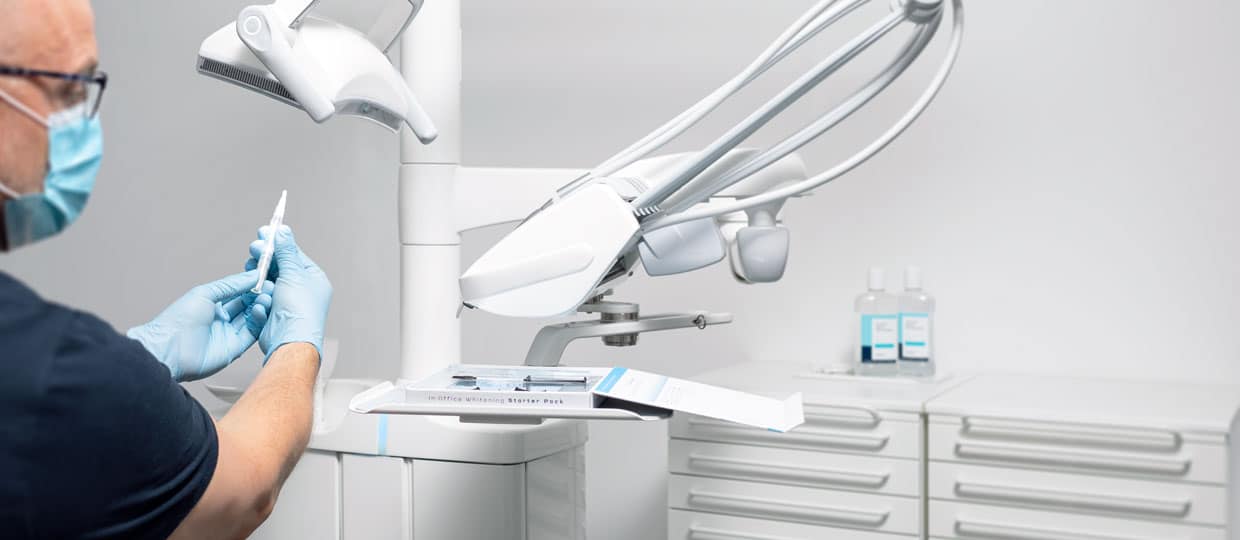
Study: The Performance of 25% Hydrogen Peroxide
Saying you have incredible products is one thing, but proving it is not always easy. Cavex had the chance to be involved in a study of the Boston University, where multiple whitening products where tested and measured on their effectiveness. In this blog we want to showcase you the test results and what this tells us about our whitening products.
Read more
About us
Cavex is there for the dental professional and their suppliers to help them in their daily work with reliable, top quality products.
Read more